What Is ClinicalTrials.gov and Why Does It Matter?
Clinical trials are a fundamental part of how modern medicine advances, yet they often feel distant or opaque to the public. Many people associate them with experimental drugs or complex laboratory research, without realizing how visible and regulated clinical research has become. One of the most important tools for that visibility is ClinicalTrials.gov, a public database designed to make medical research more transparent and accessible.
ClinicalTrials.gov is an online registry and results database of clinical studies involving human participants. It is operated by the U.S. National Library of Medicine, which is part of the National Institutes of Health. The site was launched in the year 2000 and has grown into one of the most comprehensive sources of information about clinical research anywhere in the world.
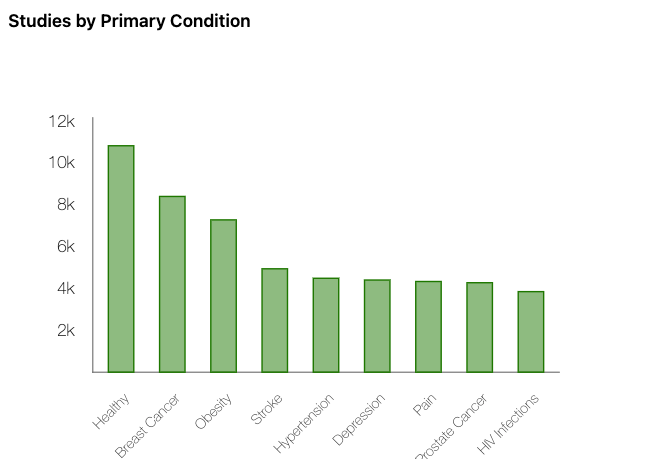
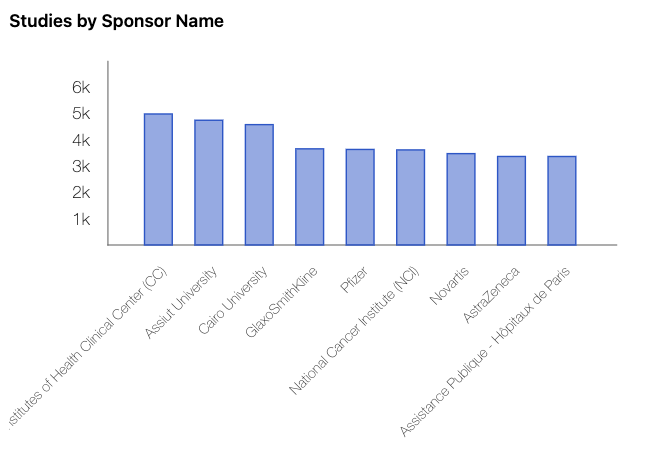
The database contains information on hundreds of thousands of studies conducted across many countries. These studies cover a wide range of medical areas, including drug treatments, medical devices, surgical procedures, behavioral therapies, diagnostic tools, and preventive strategies. Some trials involve only a small number of participants, while others include tens of thousands of people across multiple continents.
One of the main reasons ClinicalTrials.gov exists is to improve transparency in medical research. In the past, it was difficult for the public to know which clinical trials were being conducted, and some studies were never published if their results were negative or inconclusive. This made it harder to evaluate how effective or safe certain treatments truly were. ClinicalTrials.gov helps address this problem by making trial information publicly available from the start.
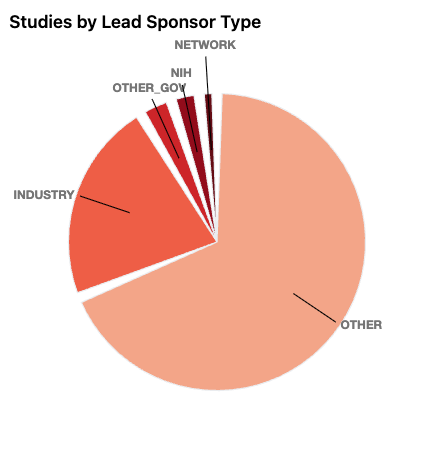
The site also plays an important role in accountability. Many clinical trials, especially those involving drugs or devices regulated by the U.S. Food and Drug Administration, are legally required to be registered on ClinicalTrials.gov. In many cases, study sponsors are also required to post summary results after the trial is completed. This reduces the risk that unfavorable findings will be quietly ignored or hidden from view.
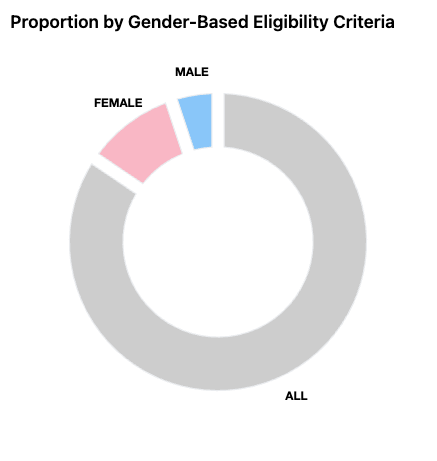
Each study listed on ClinicalTrials.gov includes a structured record that follows a standardized format. These records usually describe the purpose of the study, the medical condition being investigated, and the type of intervention being tested. They also include information about who is eligible to participate, where the study is being conducted, and whether the trial is currently recruiting participants.
Many records also include timelines, such as when the study started and when it is expected to finish. For completed studies, results may be available in a summarized form that includes outcome measures and information about side effects. While some sections can be technical, much of the content is written to be understandable to non-experts.
Patients and caregivers are among the most important users of ClinicalTrials.gov. People with serious or rare conditions often use the site to search for trials that might offer access to new treatments. Others use it to learn more about ongoing research related to their diagnosis, even if they do not plan to participate in a study.
Researchers and clinicians rely on ClinicalTrials.gov for different reasons. The database helps them identify ongoing studies, understand how similar research has been designed in the past, and avoid unnecessary duplication of work. It also provides a broader view of the evidence in a given field, including studies that may not have been published in academic journals.
ClinicalTrials.gov is also valuable to policymakers, regulators, journalists, and members of the general public. It allows independent observers to track trends in medical research, monitor compliance with reporting rules, and evaluate claims about new medical breakthroughs. By making this information public, the database supports informed discussion and evidence-based decision-making.
Clinical trials themselves are often described in terms of phases, which reflect how far along a study is in testing a medical intervention. Early-phase trials focus mainly on safety, while later-phase trials examine effectiveness in larger populations. ClinicalTrials.gov helps users understand where a study fits in this process, though not all studies follow the traditional phase structure.
It is important to understand what ClinicalTrials.gov is not. The database does not recommend treatments, endorse specific therapies, or replace advice from healthcare professionals. A trial’s presence in the database does not mean a treatment is proven to work or is safe for general use. Many studies fail, and that information is just as valuable as success.
The inclusion of results data is one of the most powerful features of ClinicalTrials.gov. By requiring certain trials to post results regardless of outcome, the database helps reduce publication bias. This ensures that medical decisions are informed by the full range of evidence, not just the most favorable findings.
Although ClinicalTrials.gov is run by a U.S. government agency, its reach is global. Researchers and organizations from around the world register their studies on the platform. As a result, it has become a central hub for international clinical research and a model for transparency initiatives in other countries.
Even people who never visit the website benefit from its existence. The medications, vaccines, and treatment guidelines used in everyday healthcare are shaped by the research documented there. By making that research visible and accountable, ClinicalTrials.gov helps build trust in the medical system.
In many ways, ClinicalTrials.gov is a quiet but essential part of modern medicine. It does not attract much attention, yet it plays a crucial role in how medical knowledge is created, evaluated, and shared. Understanding what it does helps demystify clinical research and highlights the importance of openness in advancing human health.